Scientists from USTC discover a new biomarker for early diagnosis of Alzheimer’s disease, not only helping early diagnosis in clinic but also promoting drug trials
In the upcoming 2017 of Biological Psychiatry online (IF=11.212), scientists reported that detecting the activity of the enzyme beta-secretase (β-Site Amyloid Precursor Protein–Cleaving Enzyme 1, BACE1) in the blood could predict the development of Alzheimer’s disease (AD) at an early clinical stage as well as the progression of the disease from the first symptoms of mild cognitive impairment (MCI) to AD dementia. An international team of researchers led by Professor Yong Shen, University of Science and Technology of China, Neurodegenerative Disease Research Centre (NDRC) in Hefei, China, Professor Rena Li, Director of Center for Hormone Advanced Science and Education, Roskamp Institute, Florida, United State, and Professor Harald Hampel of Sorbonne Universities, Pierre and Marie-Curie University (UPMC) and AXA Research Fund and UPMC Chair on Alzheimer's disease, Paris, France, confirmed that both MCI and AD individuals have a higher expression of the BACE1 protein in blood than in healthy controls, and that the enzymatic activity of BACE1 rises as disease worsens.
The researchers propose that blood BACE1 activity could become an effective biological marker of AD progression, which may represent a useful tool for current clinical AD therapy trials. “The difference between AD patients and controls is dramatic and the enzymatic activity increases over time being associated with disease development. This is compelling”, said Dr. Yong Shen, University of Science and Technology of China, Hefei, China, who was the leading author in the study.
Dr. Rena Li from Roskamp Institute, the corresponding author of this study, also commented that “our study is the first one to use blood-based biomarker to reliably track AD progress.
Generally, BACE1 is thought to be central in AD pathophysiology. It is highly expressed during development, as well as in diseased neurons. Processing amyloid precursor protein (APP) through this enzyme contributes to Ab production and amyloid plaque formation in AD brains. Shen, Li and Hampel and collaborators previously reported elevated levels of BACE1 in brains and cerebrospinal fluid (CSF) of patients with AD (Yang et al., Nature Medicine, 2003; Zhong et al., JAMPA Psychiatry, 2007). In the current study, Shen and his colleagues found elevated blood concentrations of BACE1 also during the progression of the disease in AD patients and MCI subjects. [Courtesy of Shen et al., 2017. ©Biological Psychiatry.]
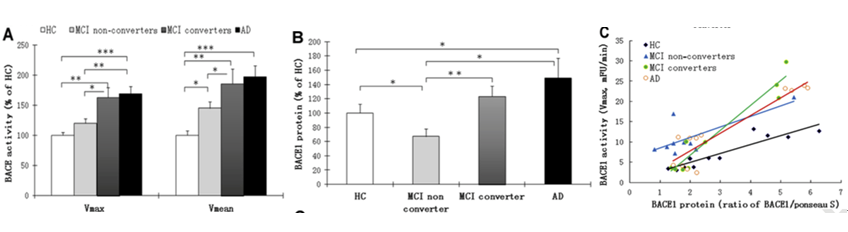
In particular, Shen and his colleagues performed the study on a total of 224 individuals recruited from three independent international academic AD research centers and memory clinics (recruited in the US, Germany and Sweden). Three age-matched multi-site study cohorts were assembled as follows: 75 probable AD patients, 96 MCI subjects, and 53 age- and sex-matched healthy controls.
They showed that blood (plasma) BACE1 activity was significantly increased in MCI subjects, primarily in individuals who progressed to AD, and in subjects with probable AD. The increase of BACE1 activity in A patient was also correlated with a worsening on the AD clinical cognitive rating scale, namely the Mini-Mental State Examination (MMSE) score. “Our results suggest that BACE1 activity may complement the evolving array of AD biomarkers for early detection, diagnosis and dissection of AD and can be a powerful tool to track disease progression”, saida senior author of the study Dr. Harald Hampel, Pierre and Marie-Curie University, Paris, France. Dr. Hampel further commented that “BACA1 as a blood biomarker could also serve as an outcome for current trials on BACE-inhibitors in AD and therefore optimize the design of critical therapy trials to enhance their chance to be successful”.
Instead of utilizing CSF for the diagnosis of AD, the ability of investigating blood biological alterations related to the development of AD offers a relevant advantage. “It is exciting that such a promising biomarker for AD diagnosis, showing the potential for monitoring disease progression, might have a key role for assessing the efficacy of therapies, especially given the recent multiple clinical trials failures we are facing” said Harald Hampel. “If the level of BACE1 activity changes dynamically in response to treatments slowing the neurodegeneration process, monitoring blood BACE1 activity might represent a potential pharmacodynamic indicator”, said Yong Shen.
Yong Shen, Harald Hampel, and colleagues are working with the Clinical Neuroscience Research in AD consortium to reproduce this data in a larger cohort. The consortium’s Phenotype-Genotype-Biomarker study collects blood and CSF samples from AD patients and MCI subjects every six to twelve months while clinically assessing cognitive and behavioral symptoms. In the next step, they will use the Paris monocenter INSIGHT-preAD study to investigate asymptomatic preclinical at risk individuals stratified by amyloid PET.
“With more than300 individuals examined longitudinally, the study will be able to determine whether BACE1 activity can predict future disease initiation, progression and conversion to first symptoms as well as rates of functional decline”, Yong Shen said. Rena Li added that BACE1 activity might be “a useful antecedent marker in people with suspected familial AD”. Harald Hampel concluded that “BACE1 in blood will be a core biomarker candidate in our newly established Alzheimer Precision Medicine Initiative (APMI) and it’s related cohort program (APMI-CP), supporting a more successful drug development in AD”.
Back
