New Approach to Characterize the Protein-Ligand Interaction Mode in the Limit of NMR Intermediate Exchange
]
Nuclear magnetic resonance (NMR) spectroscopy is an alternative and fruitful approach to depict the protein-ligand interaction modes. However, severe line broadening beyond detection of interface resonances that comes along has been a problem. Recently, Prof. RUAN Ke’s group of the University of Science and Techonology of China (USTC) established a new approach using pseudocontact shifts to fill this gap of NMR characterization.
Protein-ligand interaction modes are at the heart of the rational drug discovery campaign. High-throughput screening normally identifies initial lead compounds with micromolar affinities that are also central to fragment-based drug discovery. The structure-guided optimization of lead compounds requires high-resolution complex crystal structures, but their availability is often limited by the dynamic nature of the targets and/or the low aqueous solubility of the lead compounds. NMR spectroscopy is an alternative and fruitful approach to depict the protein-ligand interaction modes. The lead-like compounds with approximately 10 micromolar to 10 nanomolar affinities often fall into the NMR intermediate exchange scale, which can cause severe line broadening beyond detection of interface resonances. These issues thus pose an unparalleled challenge to the characterization of protein-ligand interaction modes in the NMR limit of intermediate exchange.
Prof. RUAN Ke’s group has determined the 19F low-populated bound-state pseudocontact shifts (PCSs) of mono- and di-fluorinated inhibitors of the BRM bromodomain using a highly-skewed protein/ligand ratio. The bound-state 19F PCSs were retrieved from 19F chemical exchange saturation transfer (CEST) in the presence of the lanthanide-labeled protein, which was termed the 19F PCS-CEST approach. These PCSs enriched in spatial information enabled the identification of best-fitting poses, which agree well with the crystal structure of a more soluble analog in complex with the BRM bromodomain. This work was published on Angewandte Chemie International Edition entitled as “Use of Fluorine Pseudocontact Shifts to Characterize the Protein-Ligand Interaction Mode in the Limit of NMR Intermediate Exchange” on August 28th.
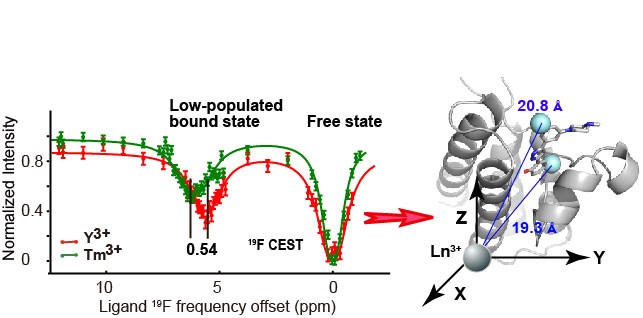
The 19F PCS-CEST approach to retrieve the low-populated bound-state 19F pseudocontact shifts, which enable the identification of the best binding pose of the BRM bromodomain inhibitor. (Image by RUAN Ke)
This approach fills the gap of the NMR structural characterization of lead-like inhibitors with intermediate affinities, which are essential for structure-guided hit-to-lead evolution.
Graduate students GAO Jia from USTC and LIANG E from Tsinghua University contributed equally to this work.This work was supported by CAS, MOST of China, National Natural Science Foundation of China.
Source:http://onlinelibrary.wiley.com/doi/10.1002/anie.201707114/full
(Hefei National Laboratory for Physical Sciences at the Microscale, School of Life Sciences)
Back
