Understanding of Long Noncoding RNA in Cell Cycle May Provide Target Position for Cancer Treatment
- Precise regulation of the cell cycle is critical to cell growth, and any tiny problem in the system may lead to tumorigenesis. Once cells pass through the G2/S checkpoint, they can only divide, thus the G1/S transition point is a vital checkpoint in the cell cycle progression. Cyclin D1/CDK4 complex is a critical regulator of cell cycle G1/S phase. Although the mechanism by which Cyclin D1/CCND1 is regulated at various levels of gene expression have been reported, it is seldom reported how widely non-coding RNAs, especially long non-coding RNAs (lncRNAs), regulate the expression of Cyclin D1/CCND1 in cells.
Researchers discovered a lncRNA called lncRNA-Assisted Stabilization of Transcripts (LAST) activated by c-Myc transcription, which can stabilize CCND1 mRNA, therefore leading to an increasing in the amount of Cyclin D1 protein and promoting more rapid cell cycle operation and tumorigenesis. The work was done by research team led by Professor WU Mian from University of Science and Technology of China (USTC) and published in the world-renowned eLife journal, titled as “LAST, a c-Myc-Inducible long noncoding RNA, cooperates with CNBP to promote CCND1 mRNA stability in human cells”.
The research reports the isolation and characterization of the novel c-Myc-regulated LAST, which acts as a CCND1 mRNA stabilizer. LAST can guide the RNA binding protein CNBP to co-bind with 5’UTR of CCND1 mRNA to In addition, the study also found that LAST/CNBP could regulate the stability of a series of other mRNA through a similar mechanism. This study, for the first time, elucidats the mechanism by which lncRNA regulates the stability of CCND1 mRNA and proves that LAST is a non-coding RNA promoting cancer, which provides a potential target for the diagnosis and treatment of related cancers.
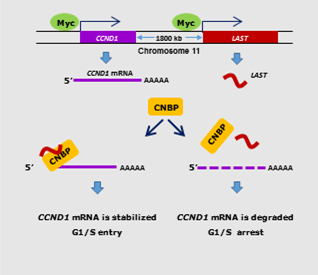
A schematic illustration of the proposed model depicting the role of c-Myc-induced LAST in regulating CCND1 mRNA stability via CNBP (Image by WU)
Professor WU Mian from USTC is the corresponding author. CAO Limian and ZHANG Pengfei, doctoral students from the research group are the co-authors of this paper. The research was supported by grants from the National Key R&D Program of China and National Natural Science Foundation of China.
The link of the paper: https://www.ncbi.nlm.nih.gov/pubmed/29199958
(DENG Weiting, USTC News Center)
Back
