Molecular Basis for the Assembly of RuBisCO Assisted by the Chaperone Raf1
A team led by Prof. ZHOU Congzhao and Prof. CHEN Yuxing from University of Science and Technology of China (USTC) reported the crystal structures of Raf1 from cyanobacteria Anabaena sp. PCC 7120 and its complex with RuBisCO large subunit RbcL, and proposed a putative model for the assembly of cyanobacterial RuBisCO coordinated by the chaperone Raf1. The series of research results were published in Nature Plants on May 25th.
Photosynthesis is a fundamental biological process on Earth that provides the energy and sugars for most living organisms. In this process, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), which is the most abundant enzyme in nature, is responsible for converting the atmospheric CO2 into organic carbon. The folding and assembly of RuBisCO needs a series of chaperones, including the RuBisCO accumulation factor Raf1, which is highly conserved in cyanobacteria and plants.
The RuBisCO enzymes are generally classified into three forms. Form I, which is the most common form widespread in plants, algae, cyanobacteria and proteobacteria, is composed of eight large (RbcL, ~53 kDa) and eight small (RbcS, ~15 kDa) subunits, whereas form II and form III RuBisCO consist of only one or more RbcL dimers. Notably, RuBisCO is considered to be inefficient owing to its slow catalytic rate and unavoidable inhibition by O2, which impairs its CO2/O2 specificity. Thus, engineering RuBisCO to improve the carboxylation efficiency and CO2/O2 specificity is viewed as a promising strategy to increase crop growth and yield.
In the research, structural analyses and biochemical assays revealed that each Raf1 dimer captures an RbcL dimer, with the C-terminal tail inserting into the catalytic pocket, and further mediates the assembly of RbcL dimers to form the octameric core of RuBisCO. Furthermore, the cryo-electron microscopy structures of the RbcL–Raf1–RbcS assembly intermediates enabled them to see a dynamic assembly process from RbcL8Raf18 to the holoenzyme RbcL8RbcS8. In vitro assays also indicated that Raf1 can attenuate and reverse CcmM-mediated cyanobacterial RuBisCO condensation.
In all, the researchers presented the crystal structures of the full-length Raf1 and its complex with RbcL, in addition to the cryo-electron microscopy (cryo-EM) structures of a series of assembly intermediates that eventually form the RuBisCO holoenzyme. Structural analysis combined with biochemical assays enabled them to figure out the fine molecular mechanism of Raf1-assisted cyanobacterial RuBisCO assembly.
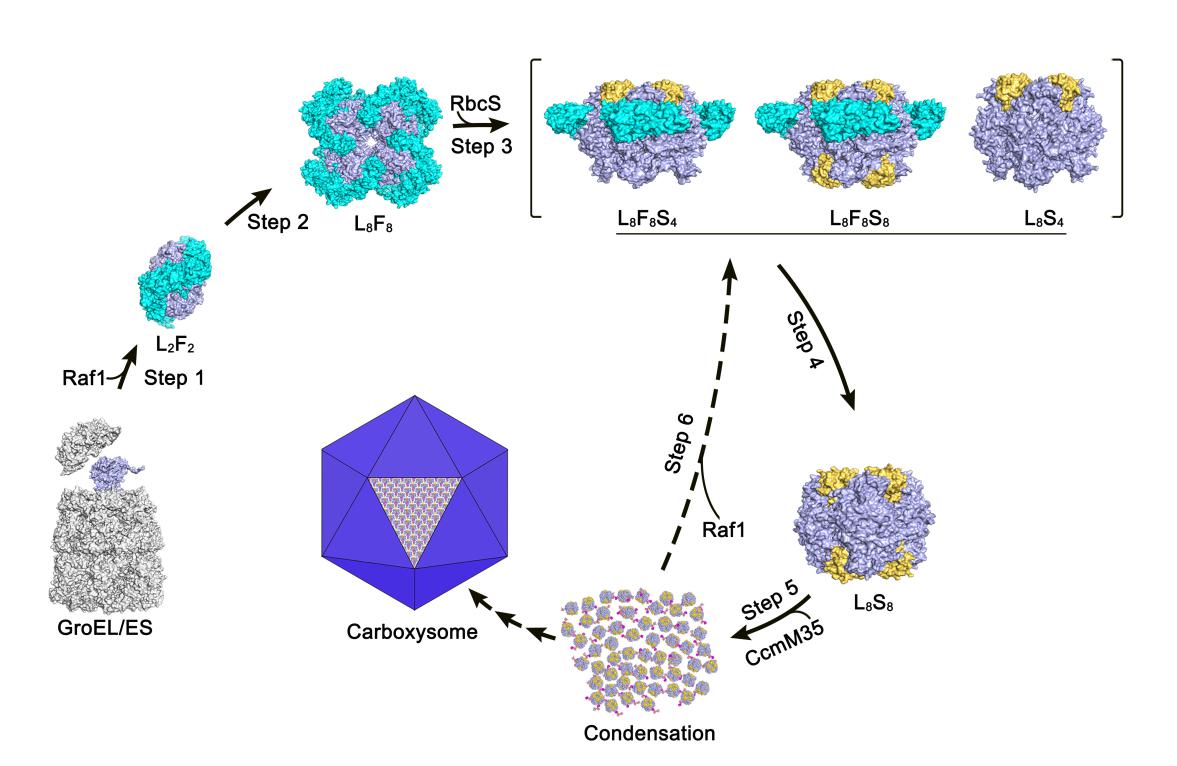
A putative model of chaperone-assisted assembly of cyanobacterial RuBisCO (Image by XIA Lingyun et al.)
Paper link: https://www.nature.com/articles/s41477-020-0665-8
(Written by LI Xiaoxi, edited by JIANG Pengcen, USTC News Center)
Back
