Researchers Reveal a New Inhibitory Mechanism Against Bacterial DNA Replication
Previous studies reported that all of the discovered β-clamp inhibitors and binding proteins engaged the β-clamp via hydrophobic protein-binding pocket. In a recent study led by Prof. ZHANG, however, has found that such hydrophobic protein-binding pocket is not a must.
The research team reported a cryo-EM structure of the clamp-Gp168 complex at 3.2-A° resolution. At such high resolution, researchers obtained the structural details of the complex. They found that the Gp168 dimer occupied the DNA sliding channel of β-clamp and blocked its loading onto DNA.
To understand the formation of the Gp168-clamp complex, researchers identified that Gp168 existed as a hexameric complex in solution. The pulled-down experiment involving Gp168 and the β-clamp of Staphylococcus aureus showed that Gp168 could form a stable complex with the β-clamp of Staphylococcus aureus. Researchers then performed the cryo-EM single-particle analysis to further understand the detailed structure of the Gp168-clamp complex.
Based on the results, they concluded that Gp168 represented a new class of β-clamp inhibitor with other members yet to be discovered.
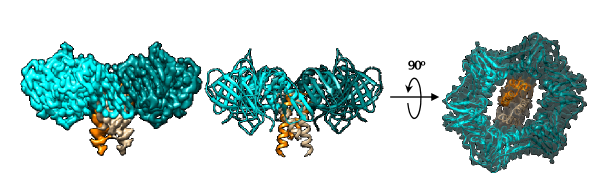
Cryo-EM structure of the clamp-Gp168 complex (Image by LIU Bing et al.)
(Written by ZHANG Yuxin, edited by LI Xiaoxi, USTC News Center)
Back
